NB 6-2 details
Videos
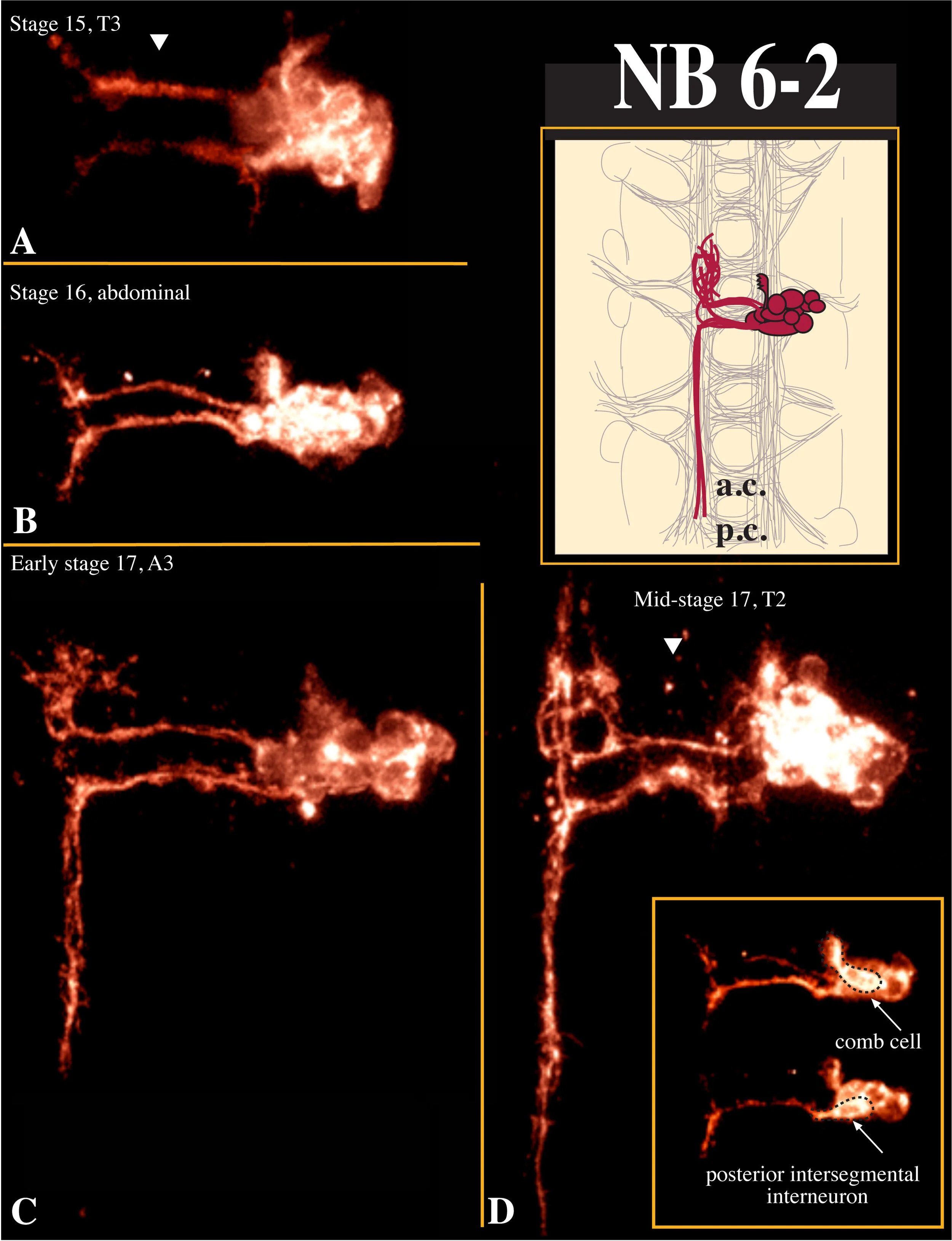
NB 6-2 delaminates at S2 in the intermediate column.
No information about the lineage derived from NB 6-2 is available from other insects.
At S2, NB 6-2 expresses engrailed (en) and intermediate neuroblasts defective (ind) (Doe 1992; Broadus et al, 1995; Weiss et al, 1998). seven-up-lacZ (svp-lacZ) and gooseberry distal (gsb-d) expression is detected by S3 (Broadus et al, 1995). At S4, Klumpfuss (Klu) is detected (Yang et al, 1997), but ind is no longer detected. By S5, NB 6-2 adds unplugged (upg) expression (Chiang et al, 1995).
Its lineage was described by Bossing et al. (1996) as containing 8-16 interneurons that project in two bundles across the posterior commissure.
A. Interneurons
The two most medial cells of the clone are large, dorsal, engrailed-GFP-negative intersegmental interneurons (6.5 um; n=16).They project across the posterior commissure in an anterior fascicle and then extend anteriorly. A second pair of very large cells (10 x 3.8 um; n=14) are ventral and engrailed-GFP-positive; the more posterior of the pair is an intersegmental interneuron that projects across the posterior commissure in a posterior fascicle and then extends posteriorly. The more anterior of the pair is a local interneuron we call the "comb cell" because it has a distinctive short anterior ipsilateral projection with all of its synaptic contacts forming on the medial side of the process. It is remarkable that these two cells share a number of distinctive features (engrailed-GFP-positive, large oblong cell shape, ventral position), yet make such different projections (local ipsilateral vs. intersegmental contralateral). The remaining local interneurons are round and varied in size (2.0-6.5 um; n=35). They project across the posterior commissure and extend anteriorly and posteriorly. The anterior projection ends in a complex meshwork similar to that observed in the NB 1-2 clone.
References:
Bossing, T., Udolph, G., Doe, C. Q., and Technau, G. M. (1996). The Embryonic CNS lineages of Drosophila melanogaster I. Neuroblast lineages derived from the ventral half of the neurectoderm. Dev Biol 179: 41-64.
Broadus, J., Skeath, J. B., Spana, E. P., Bossing, T., Technau, G. M., and Doe, C. Q. (1995). New neuroblast markers and the origin of the aCC/pCC neurons in the Drosophila central nervous system. Mech Dev 53: 393-402.
Chiang, C., Young, K.E., and Beachy, P.A. (1995). Control of Drosophila tracheal branching by the novel homeoomain gene unplugged, a regulatory target for genes of the bithorax complex. Development 121(11):3901-12.
Doe, C. Q. (1992) Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development 116: 855-863.
Skeath, J. B., Zhang, Y., Holmgren, R., Carroll, S. B., and Doe, C. Q. (1995). Specification of neuroblast identity in the Drosophila embryonic central nervous system by gooseberry-distal. Nature 376: 427-430.
Weiss, J., VonOhlen, T., Mellerick, D., Dressler, G., Doe, C. Q., and Scott, M.P. (1998). Dorsoventral patterning in the Drosophila central nervous system: the intermediate neuroblasts defective homeobox gene specifies intermediate column identity. Genes Dev 12:3591-3602.
Yang, X., Bahri, S., Klein, T., and Chia, W. (1997). Klumpfuss, a putative Drosophila zinc finger transcription factor, acts to differentiate between the identities of two secondary precursor cells within one neuroblast lineage. Genes Dev 11(11):1396-1408.